What is Gene Therapy?
To understand the role of gene therapy in cancer treatment deep study of gene therapy is very necessary. Gene therapy is a medical treatment that uses genes to prevent or treat diseases. It involves introducing healthy copies of a gene into cells to replace faulty or missing ones, allowing cells to function normally again.
Gene therapy can be used to:
1. Replace a faulty gene with a healthy one
2. Inactivate a faulty gene
3. Introduce a new gene to help fight disease
What is Gene?
A gene is a unit of heredity that carries information from one generation to the next. It is a segment of DNA (deoxyribonucleic acid) that contains the instructions for making a specific protein or performing a specific function. Study of gene plays an important role in gene therapy for cancer treatment.
Think of a gene like a recipe book:
– The recipe book (DNA) contains many individual recipes (genes)
– Each recipe (gene) has a specific set of instructions for making a particular dish (protein)
– The ingredients and instructions in the recipe (gene) determine the final product (protein)
Genes are made up of:
1. DNA sequence: The specific order of nucleotides (A, C, G, and T) that make up the gene
2. Promoter region: The part of the gene that controls when and where the gene is turned on
3. Coding region: The part of the gene that contains the instructions for making a protein
4. Non-coding region: The part of the gene that doesn’t code for a protein but may still have regulatory functions
Genes play a crucial role in:
1. Inheritance: Passing traits from parents to offspring
2. Development: Controlling growth and development
3. Function: Determining the characteristics and functions of an organism
How Does Gene Therapy Work?
Here’s a simplified explanation of how gene therapy works in cancer treatment:
Step 1: Identifying the faulty gene
– Scientists identify the specific gene responsible for a particular disease or condition.
– They study the gene’s function and how it contributes to the disease.
Step 2: Creating a healthy copy of the gene
– Researchers create a healthy copy of the faulty gene in a laboratory.
– This healthy copy is called a “transgene.”
Step 3: Delivering the transgene
– The transgene is inserted into a delivery vehicle, such as a virus (called a vector).
– The vector is designed to target specific cells or tissues.
Step 4: Introducing the vector
– The vector is introduced into the patient’s body, usually through injection or infusion.
– The vector travels to the targeted cells or tissues.
Step 5: Expression of the transgene
– The transgene is expressed, producing a functional protein.
– The protein replaces or compensates for the faulty protein, alleviating the disease symptoms.
Step 6: Monitoring and follow-up
– Patients are monitored for safety, efficacy, and potential side effects.
– Follow-up treatments may be necessary to maintain the therapeutic effect.
Gene therapy approaches:
1. Gene replacement: Replaces a faulty gene with a healthy copy.
2. Gene editing: Repairs or modifies the faulty gene.
3. Gene silencing: Reduces or eliminates the faulty gene’s expression.
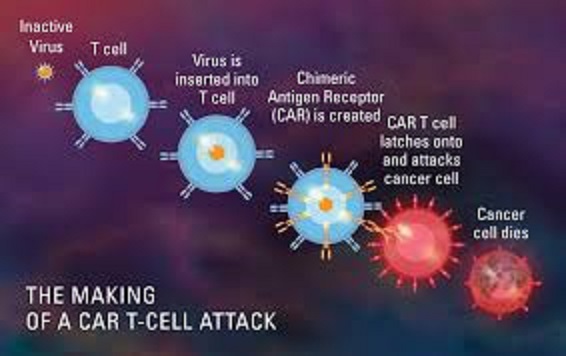
What is CAR T- cell Therapy?
There are several types of gene therapy being explored for cancer treatment:
1. Suicide Gene Therapy: Introduces a gene that makes cancer cells sensitive to a specific drug, causing cell death.
2. Immunotherapy: Stimulates the immune system to attack cancer cells by introducing genes that encode immune-stimulating proteins.
3. Gene Silencing: Uses RNA interference (RNAi) to silence genes that promote cancer growth.
4. Gene Editing: Uses CRISPR/Cas9 to edit genes that contribute to cancer development.
5. Oncolytic Virotherapy: Uses viruses that selectively infect and kill cancer cells.
6. Gene Transfer: Introduces healthy copies of a gene to replace faulty ones, slowing cancer growth.
7. Cytokine Gene Therapy: Introduces genes that encode cytokines, proteins that stimulate the immune system.
8. Tumor Suppressor Gene Therapy: Introduces genes that encode tumor suppressor proteins, which help regulate cell growth.
9. Combination Gene Therapy: Combines multiple gene therapy approaches for enhanced anti-tumor effects.
10. Targeted Gene Therapy: Delivers genes specifically to cancer cells, reducing harm to healthy cells.
These gene therapy approaches aim to selectively target and eliminate cancer cells, offering promising alternatives to traditional cancer treatments.
Steps of CAR T-cell therapy
Here are the steps involved in CAR T-cell therapy:
Step 1: Harvesting T-cells
– Collecting T-cells from a patient’s blood or bone marrow
– Isolating the T-cells using specialized equipment
Step 2: Genetic Modification
– Using viral vectors (e.g., lentivirus or retrovirus) to introduce the CAR gene into the T-cells
– The CAR gene encodes a receptor that recognizes specific proteins on cancer cells
Step 3: Expansion and Activation
– Growing and expanding the modified T-cells in a laboratory
– Activating the T-cells using chemical signals to enhance their function
Step 4: Quality Control
– Testing the CAR T-cells for purity, potency, and sterility
– Ensuring the cells meet specific criteria for infusion
Step 5: Conditioning
– Preparing the patient’s body for CAR T-cell infusion
– Administering chemotherapy or radiation to reduce tumor burden and suppress the immune system
Step 6: Infusion
– Returning the CAR T-cells to the patient’s body through an intravenous infusion
– Monitoring the patient for side effects and response to treatment
Step 7: Post-Infusion Care
– Closely monitoring the patient for side effects, such as cytokine release syndrome
– Providing supportive care, including medications and hospitalization if needed
Step 8: Follow-up
– Regularly monitoring the patient’s response to treatment and potential side effects
– Adjusting treatment plans as needed to optimize outcomes
Step 9: Maintenance Therapy
– Administering additional treatments to maintain the CAR T-cell response
– Monitoring for signs of relapse or disease progression
Note: The specific steps and details may vary depending on the institution, clinical trial, or treatment protocol.
Side effects Of CAR T-cell Therapy In cancer Treatment
Here are the side effects of CAR T-cell therapy in cancer treatment:
Common side effects:
1. Cytokine release syndrome (CRS): A potentially life-threatening condition caused by the release of cytokines.
2. B-cell aplasia: A decrease in B-cells, which can increase the risk of infections.
3. Fatigue
4. Headache
5. Nausea and vomiting
6. Muscle or joint pain
7. Fever
8. Chills
9. Swelling
10. Rash
Severe side effects:
1. Neurotoxicity: Confusion, disorientation, seizures, or cerebral edema.
2. Hemophagocytic lymphohistiocytosis (HLH): A rare but life-threatening condition.
3. Coagulopathy: Blood clotting disorders.
4. Cardiac toxicity: Heart problems, such as arrhythmias or decreased cardiac function.
5. Pulmonary toxicity: Respiratory problems, such as pneumonitis or acute respiratory distress syndrome.
6. Hepatic toxicity: Liver damage or failure.
7. Renal toxicity: Kidney damage or failure.
More About Side Effects:
Long-term side effects:
1. B-cell recovery: May take several months to several years.
2. Immune system suppression: May increase the risk of infections.
3. Secondary cancers: Rarely, CAR T-cell therapy may increase the risk of secondary cancers.
4. Gene editing errors: Off-target effects or mosaicism.
CAR T-cell specific side effects:
1. Tocilizumab resistance: Reduced effectiveness of tocilizumab in treating CRS.
2. CAR T-cell expansion: May cause CRS or neurotoxicity.
3. Antigen escape: Cancer cells may evade recognition by CAR T-cells.
Future Findings Of Gene Therapy In Cancer Treatment
Future findings of gene therapy for cancer treatment look promising, with several emerging therapies expected to revolutionize oncology in the coming years ¹. Here are some of the most exciting developments:
Immunotherapy: This approach harnesses the power of the immune system to fight cancer. It includes checkpoint inhibitors, which block proteins used by cancer cells to evade detection, and CAR-T cell therapy, which involves engineering a patient’s own T-cells to recognize and destroy cancer cells.
Precision Medicine: This approach involves tailoring treatment to individual patients based on their genetic profile. It includes genomic profiling, which analyzes the DNA of a patient’s tumor to identify specific genetic mutations, and liquid biopsies, which detect cancer-related genetic material in a patient’s blood.
Gene Editing: This approach involves making precise changes to the DNA of cancer cells to correct genetic mutations that cause cancer. It includes CRISPR-Cas9, which has already shown promise in clinical trials, and newer techniques like base editing and prime editing.
Microbiome Research: This approach involves manipulating the microbiome to enhance the effectiveness of cancer treatments. It includes microbiome modulation, which involves altering the microbiome through diet, probiotics, or fecal transplants, and probiotic cancer treatments, which involve using beneficial bacteria to improve treatment outcomes.
Overall, these emerging therapies offer new hope for cancer patients and their families, and are expected to lead to more effective, personalized, and less invasive treatments in the future .